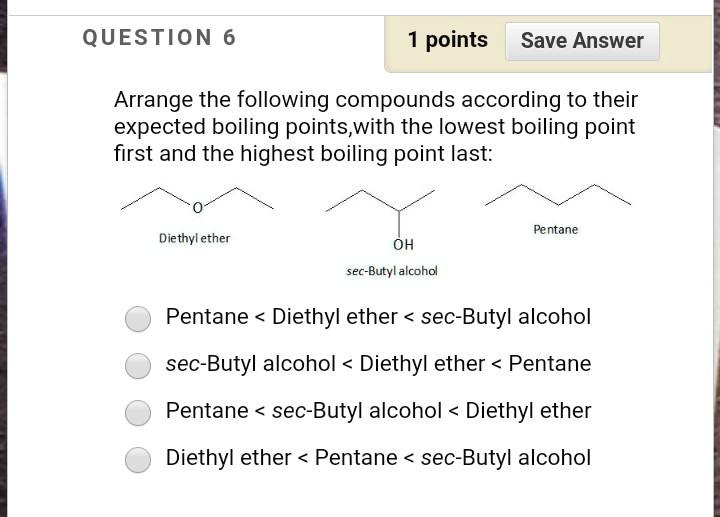
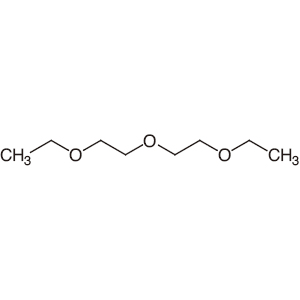
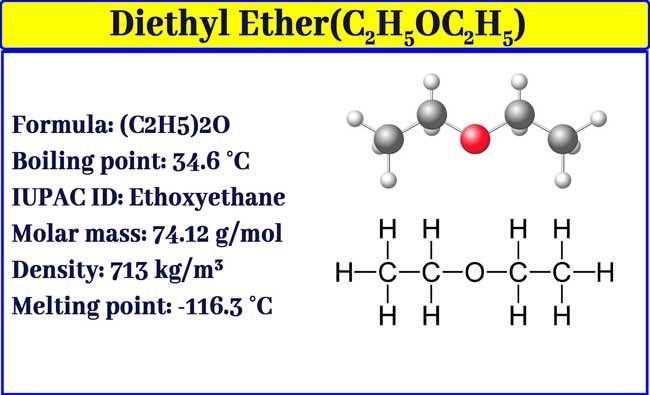
Diethyl ether has a normal boiling point of `35.0^(@)C` and has an entropy of vaporization of `84.4 - YouTube

Name the following(1) Thte non - sublimable solid from a mxiture of iodine and potassion nitrate.(2) The heavier liquid component from - mercury and water(3) The lower boiling point component from methyl

Diethyl ether - An SDS for a substance is not primarily intended for use by the general consumer, - Studocu

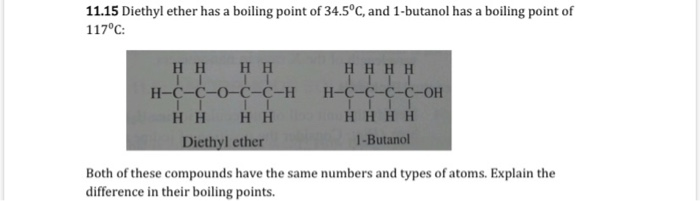
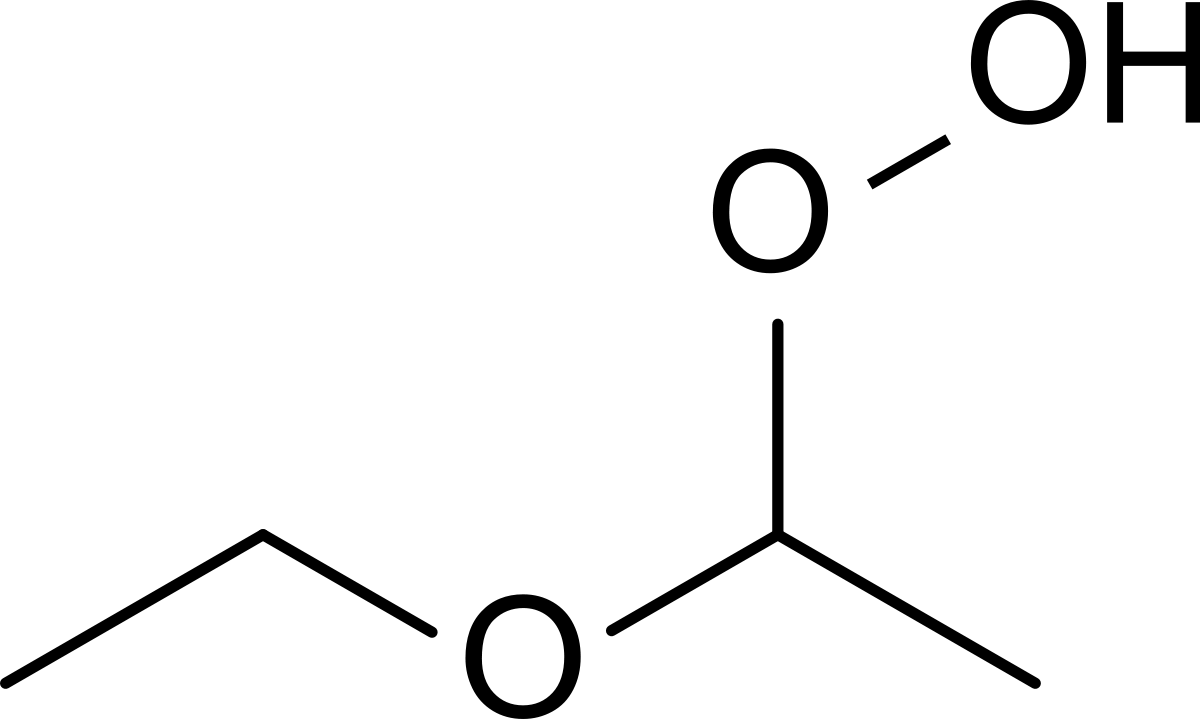
The boiling point of butan-2-one (80 C) is significantly higher than the boiling point of diethyl ether (35 C), even though both compounds exhibit dipole-dipole interactions and have comparable molecular weights. Offer
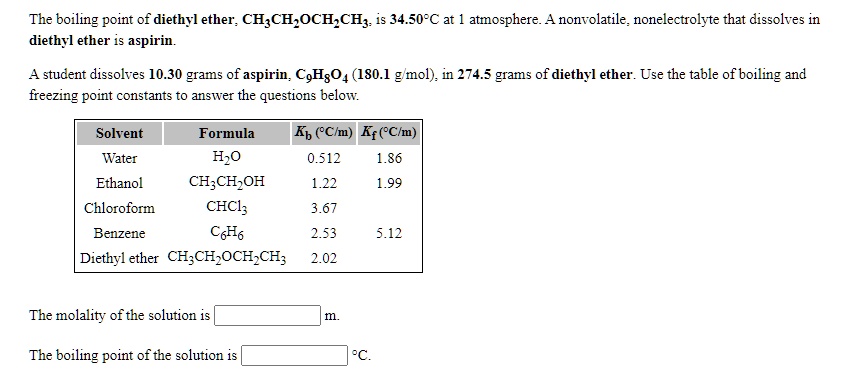
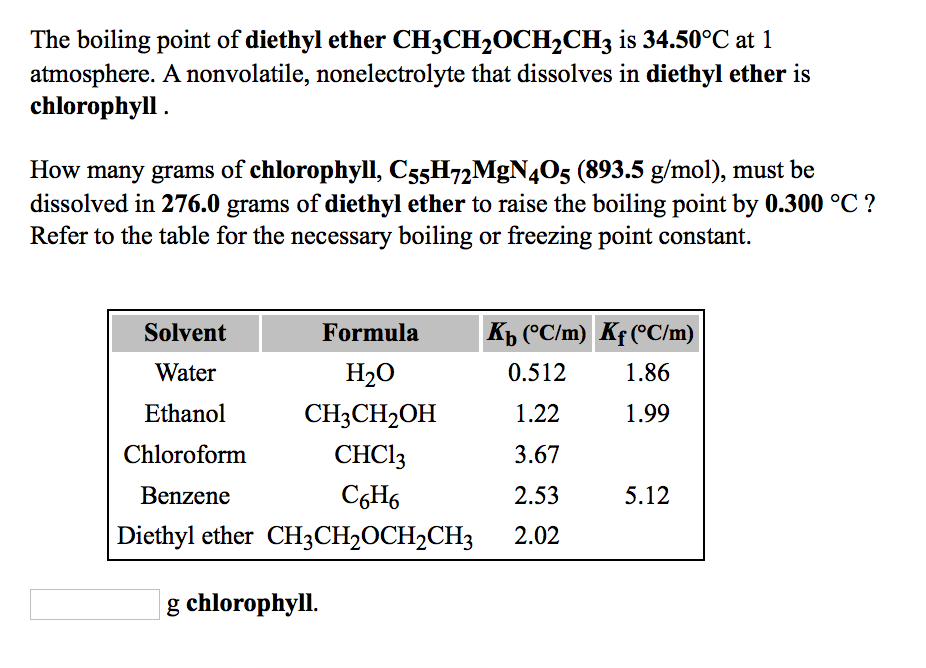
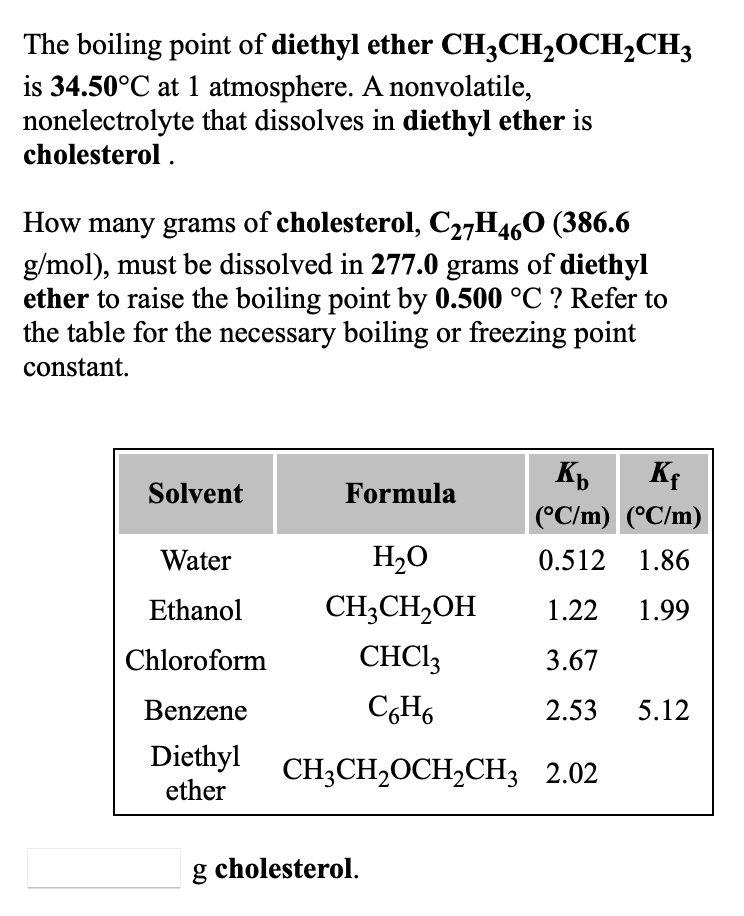
SOLVED: The boiling point of diethyl ether; CHzCH,OCH,CHz: is 34.50-C at atmosphere nonvolatile nonelectrolyte that dissolves in diethyl ether is aspirin. A student dissolves 10.30 grams of aspirin; CgHs04 (180.1 mol): in