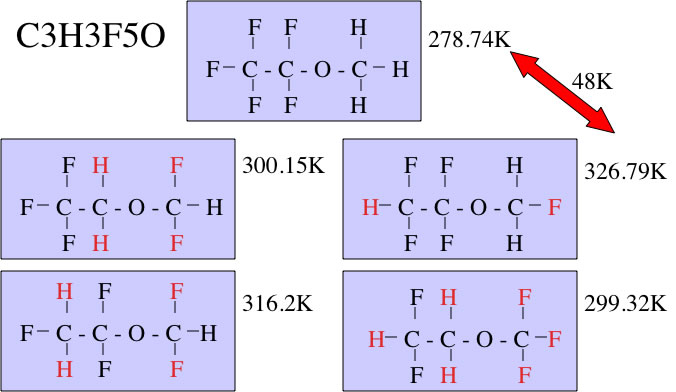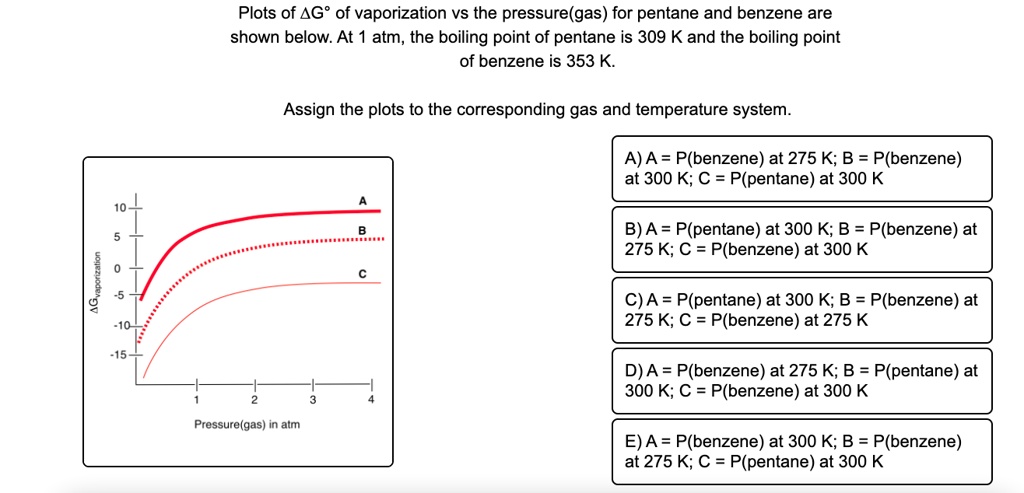
SOLVED: Plots of AG? of vaporization vs the pressure(gas) for pentane and benzene are shown below: At atm, the boiling point of pentane is 309 K and the boiling point of benzene
14. A urea solution has boiling point 373.128K then what is its melting point ? Given kf =1.86 and kb =0.52.

The normal boiling point of toluene is 110.7^(@)C and its boiling point elevation constant 3.32 " K kg mol"^(-1). The enthalpy of vaporization of toluene is nearly :

The normal boiling point of water is 373 k. vapour of waterr at temperature T is 19 mm hg. If en... - YouTube

Determine the normal boiling point in K of a substance whose vapor pressure is 55.1 mmHg at 23.2^o C and has a ?H_vap of 32.1 kJ/mol. | Homework.Study.com

Density- 2.702 g/cm^3 Melting point- 660.32 degrees C, 933.57 K Boiling point- 2466.85 Degrees C, 2740.00 K | Electron configuration, Protons, Electrons

Predicted boiling point temperature values in unit of K for selected... | Download Scientific Diagram

The normal melting and boiling points of a substance are -163 degrees Celsius and -128 degrees Celsius, respectively. Its triple point is at 125 K and 0.37 atm. Its critical point is
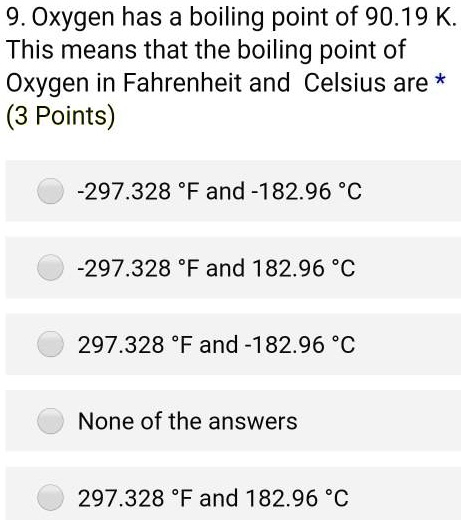
SOLVED: 9. Oxygen has a boiling point of 90.19 K This means that the boiling point of Oxygen in Fahrenheit and Celsius are * (3 Points) 2297.328 F and-182.96 %C 297.328 Fand

Temperature Temperature Scales Fahrenheit 212 o F 180 o F 32 o F Celcius 100 o C 0 o C Kelvin 373 K 100 K 273 K Boiling point of water Freezing point. - ppt download