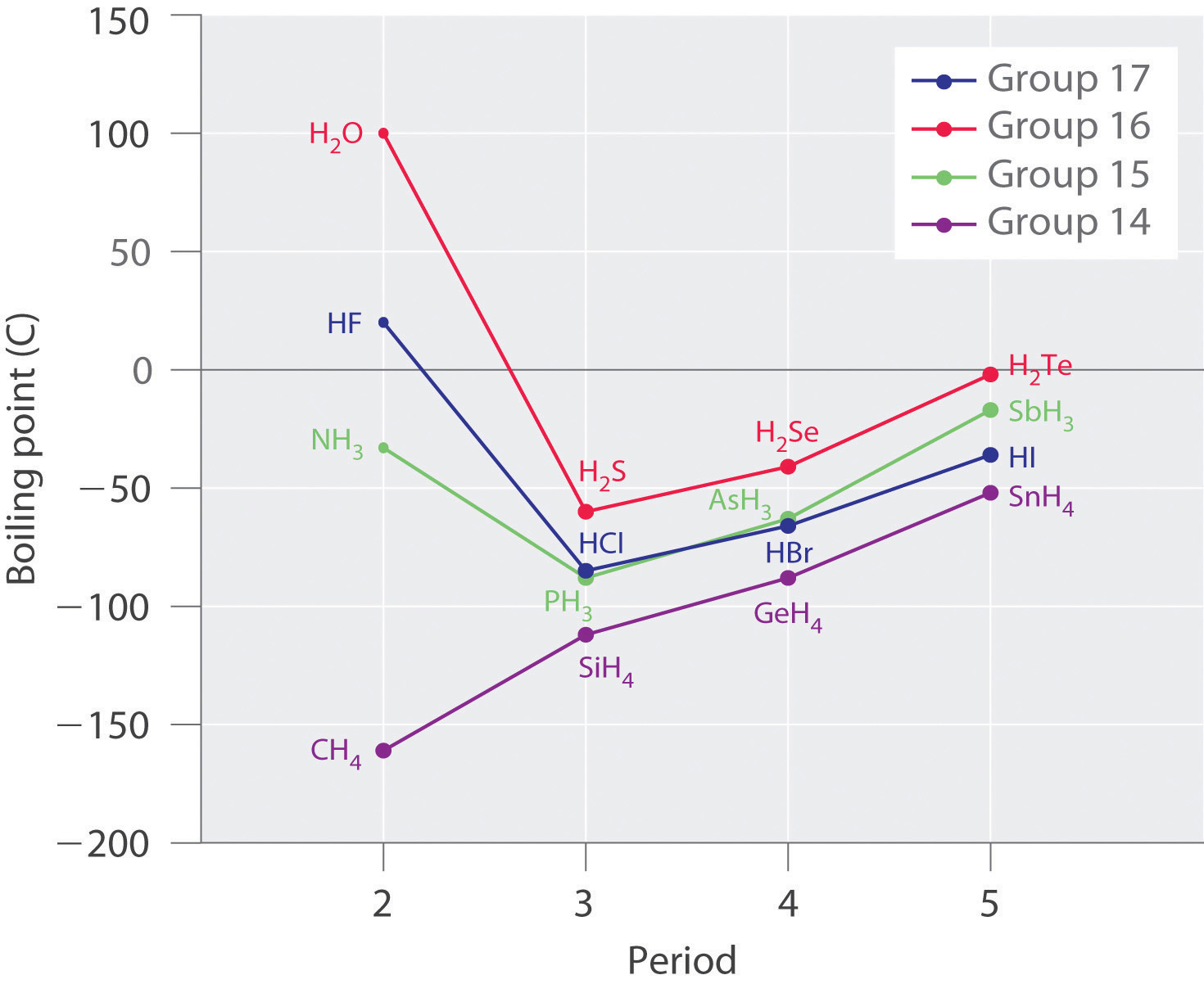
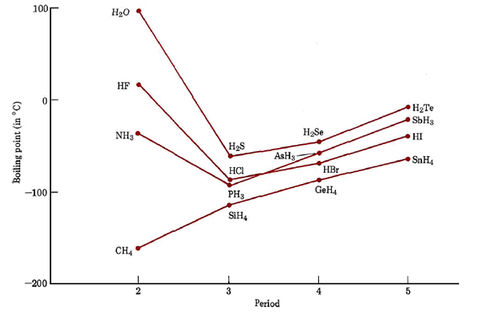
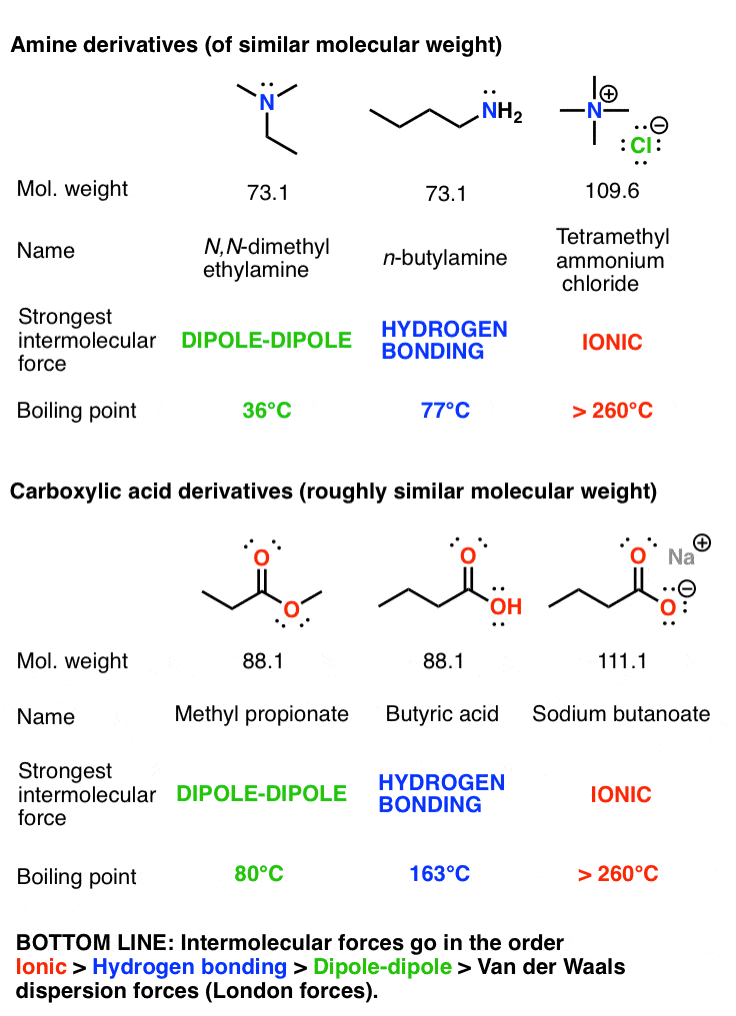
4: Boiling points of the hydrogen compounds of Groups 4A, 5A, 6A, and... | Download Scientific Diagram
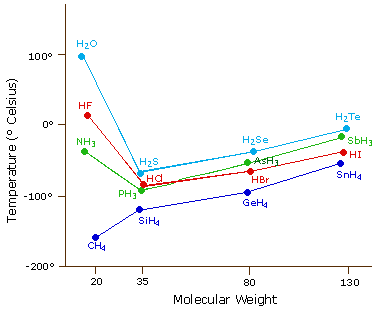
What did scientists expect as the water's boiling point without hydrogen bonds? 'It must be less than 100 degrees Celsius, but specifically equals what?' - Quora

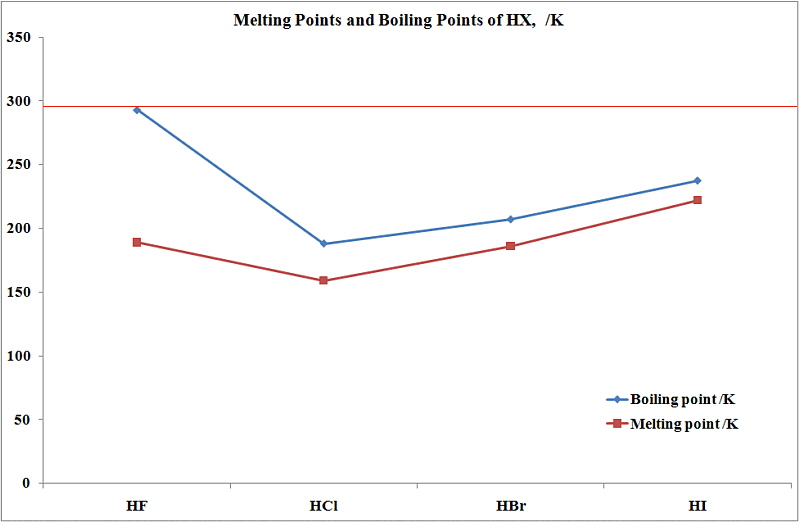
The variation of the boiling points of the hydrogen halides is in the order `HFgtHIgtHBrgtHCl`. - YouTube

Elements Hydrogen Number of: Protons 1 Neutrons 0 Electrons 1 Boiling point -252 Freezing point -259 State at room temperature gas. - ppt download
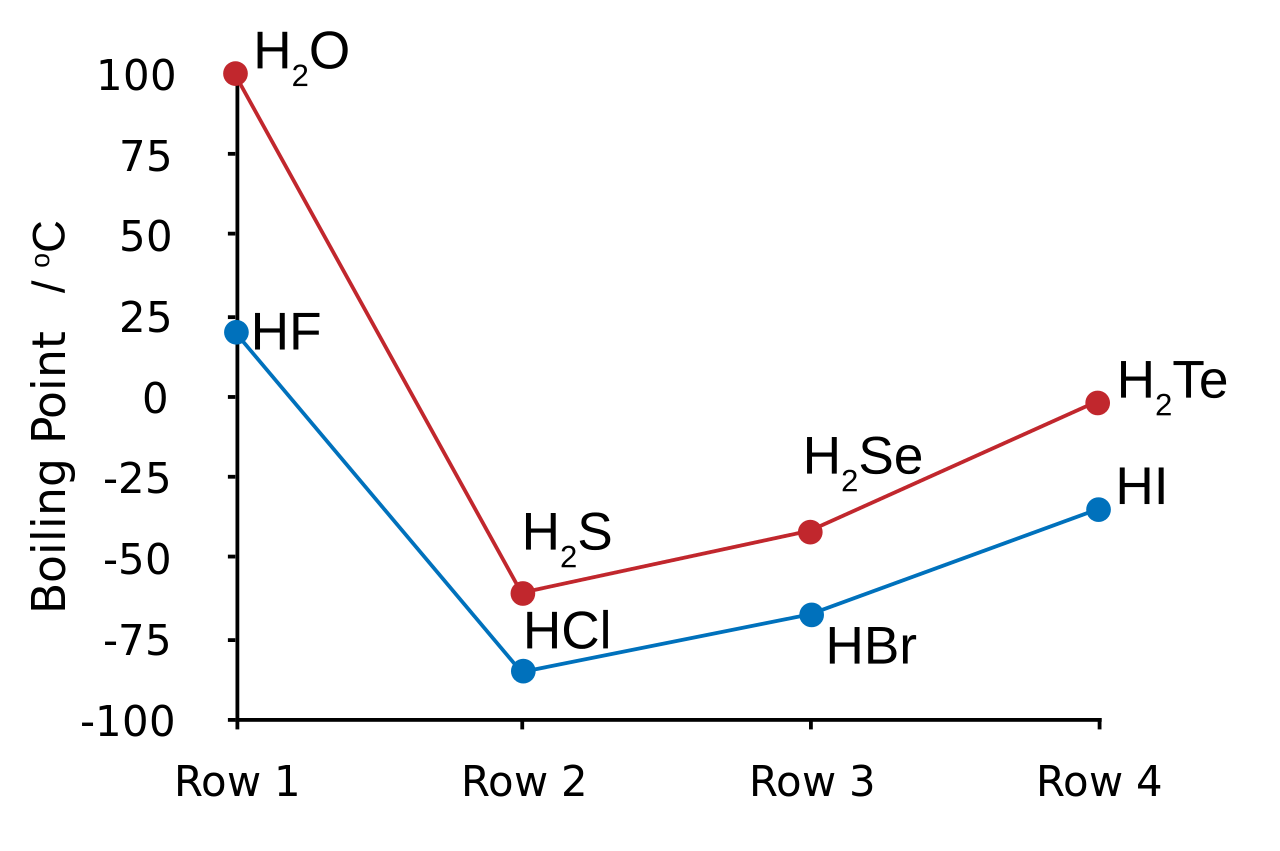
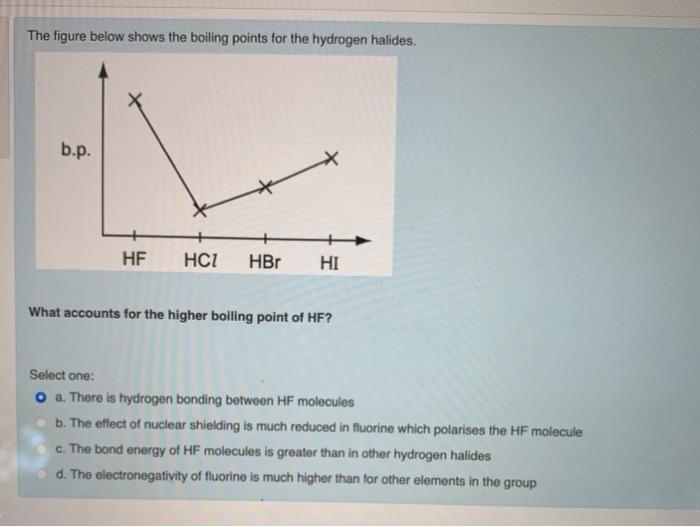
The liquefied hydrogen halides have the normal boiling points given above. The relatively high boiling point of HF can be correctly explained by which of the following? | Socratic