Latest set of Humira commercials debuts to mixed patient response - MM+M - Medical Marketing and Media
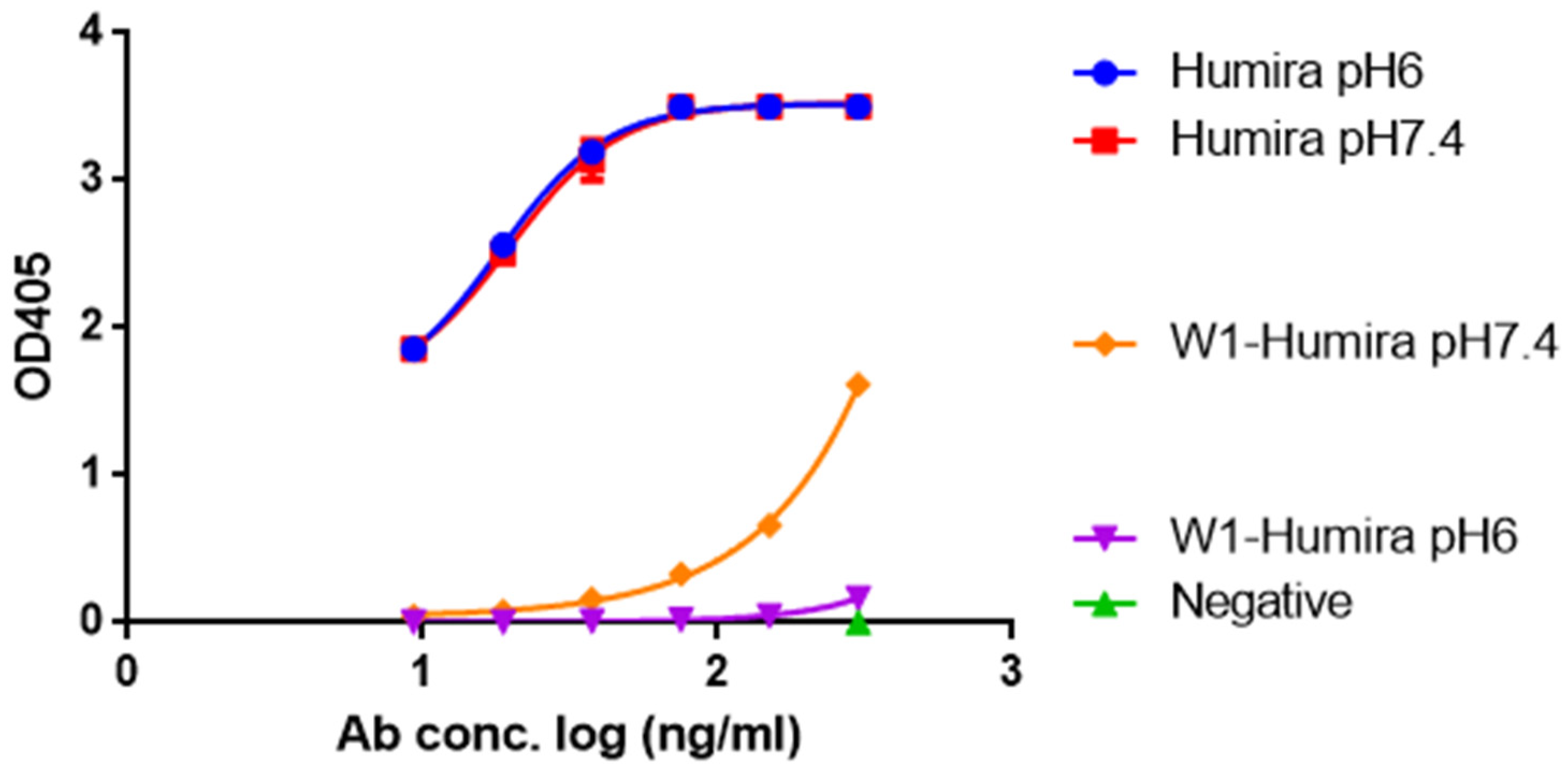
Biomolecules | Free Full-Text | Anti-TNF Alpha Antibody Humira with pH-dependent Binding Characteristics: A constant-pH Molecular Dynamics, Gaussian Accelerated Molecular Dynamics, and In Vitro Study
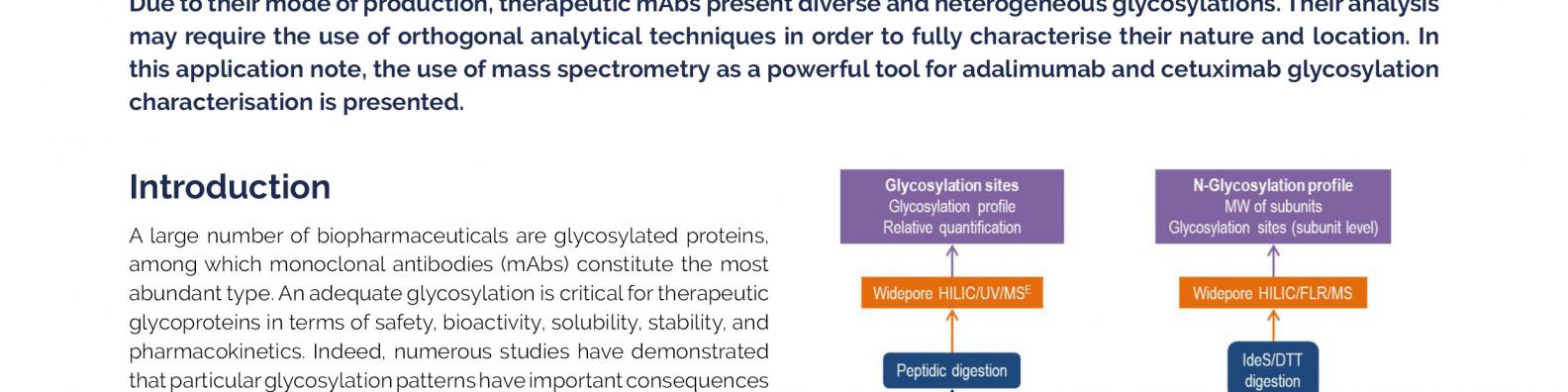
Characterisation of the glycosylation of Humira (adalimumab) and Erbitux (Cetuximab) by high resolution mass spectrometry | Quality Assistance
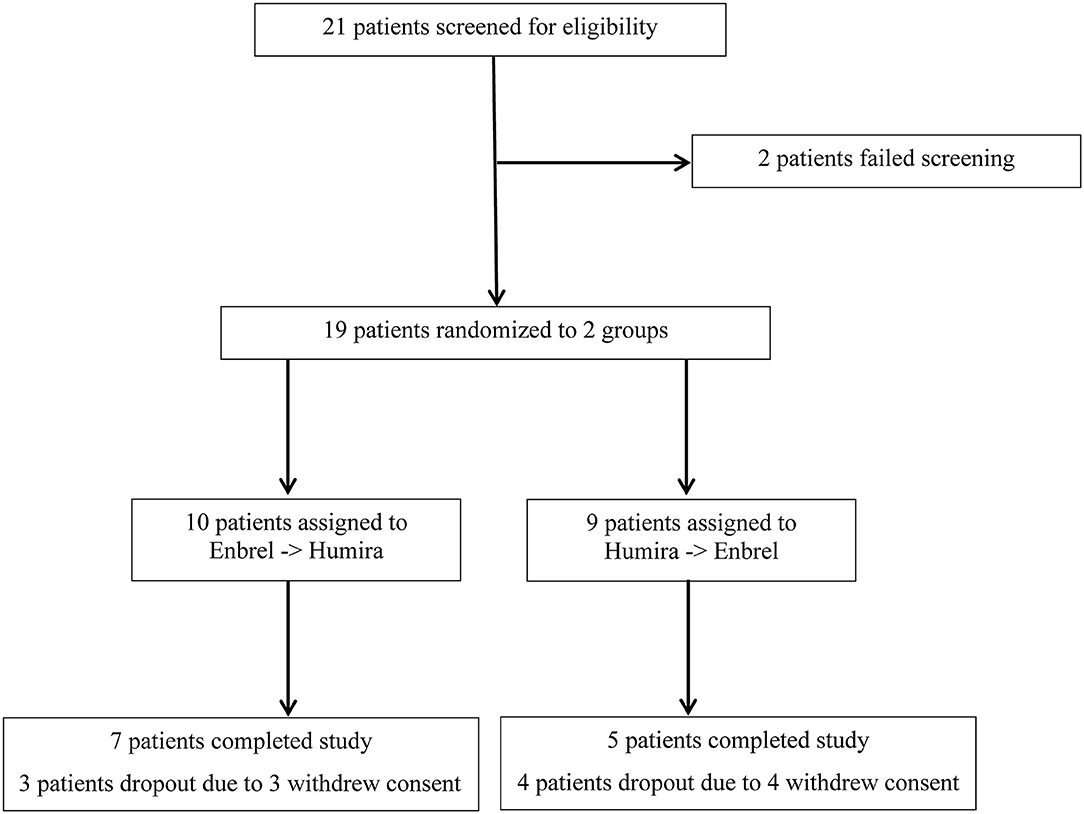
Frontiers | Head-to-Head Comparison of Etanercept vs. Adalimumab in the Treatment of Ankylosing Spondylitis: An Open-Label Randomized Controlled Crossover Clinical Trial

Figure 5 from Molecular Basis for the Neutralization of Tumor Necrosis Factor α by Certolizumab Pegol in the Treatment of Inflammatory Autoimmune Diseases | Semantic Scholar
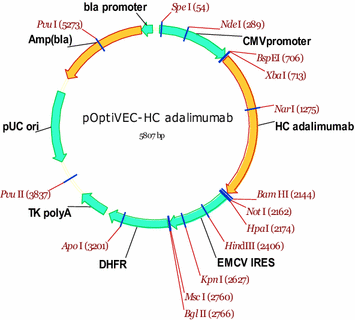
Design of a stable cell line producing a recombinant monoclonal anti-TNFα antibody based on a CHO cell line | SpringerPlus | Full Text

The amino acid sequences of abatacept, alefacept, etanercept, and H... | Download Scientific Diagram

Physicochemical and Functional Characterization of HS016, a Biosimilar of Adalimumab (Humira) - Journal of Pharmaceutical Sciences
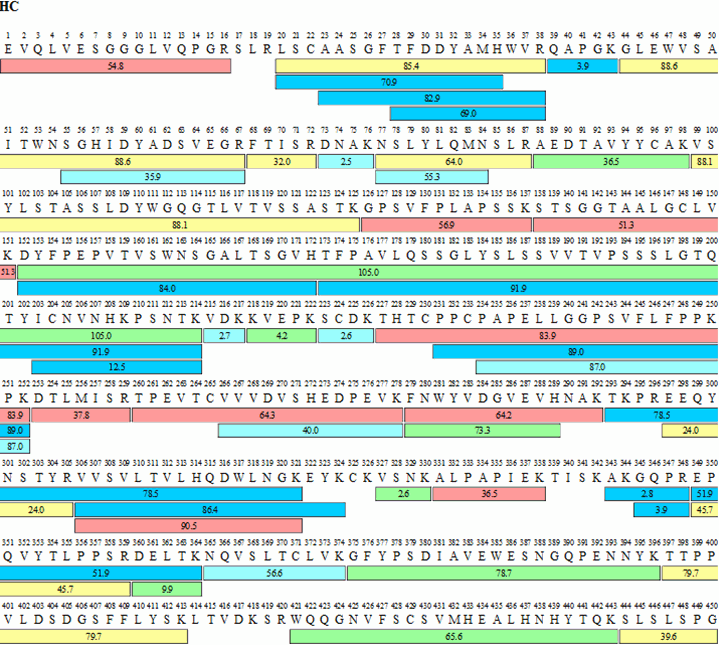
Primary structure of HLX03 and CN-Humira®. a Tryptic peptide maps; MS... | Download Scientific Diagram

HLA-DQA1*05 Carriage Associated With Development of Anti-Drug Antibodies to Infliximab and Adalimumab in Patients With Crohn's Disease - ScienceDirect

B. Space-filling models of the monoclonal antibody Adalimumab (Humira)... | Download Scientific Diagram
Comparison of the Inhibition Mechanisms of Adalimumab and Infliximab in Treating Tumor Necrosis Factor α-Associated Diseases

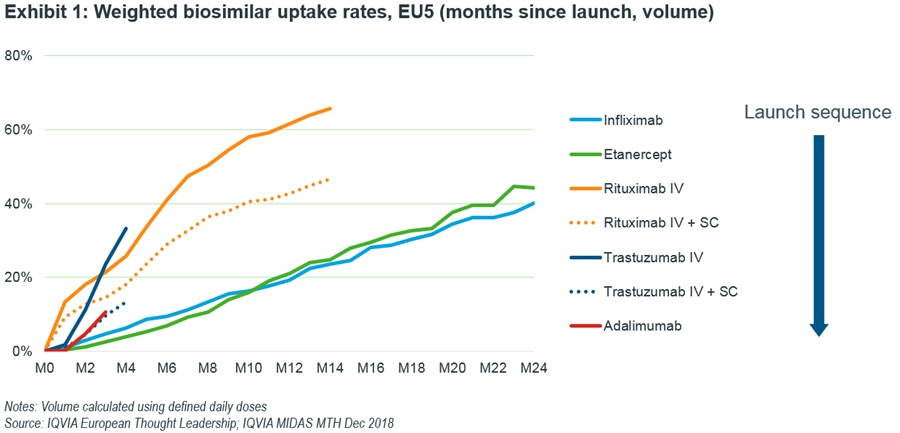
FDA Grants Interchangeable Status to Humira Biosimilar, but Certain Factors May Hamper Its, Other Adalimumabs' Uptake - MMIT Network
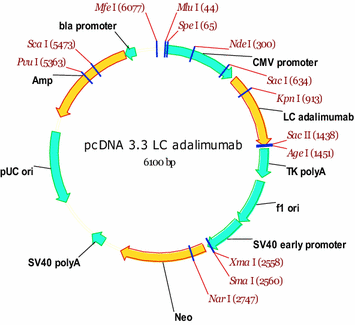
Design of a stable cell line producing a recombinant monoclonal anti-TNFα antibody based on a CHO cell line | SpringerPlus | Full Text

Amino acid sequences of adalimumab variable heavy (VH) and light chain... | Download Scientific Diagram
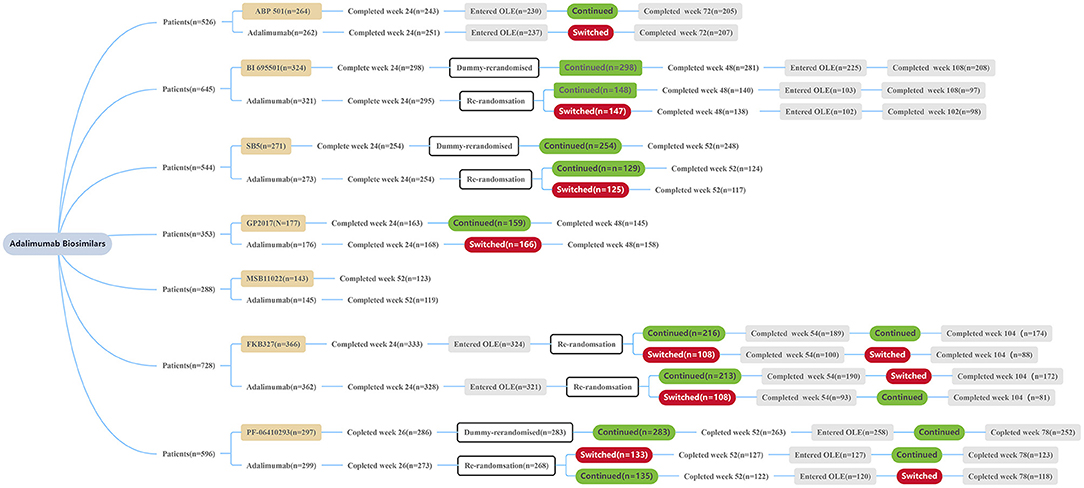
Frontiers | Efficacy and Safety of Adalimumab Biosimilars: Current Critical Clinical Data in Rheumatoid Arthritis
Patient perspectives of successful adalimumab biosimilar transitioning in Crohn's disease: an interview study
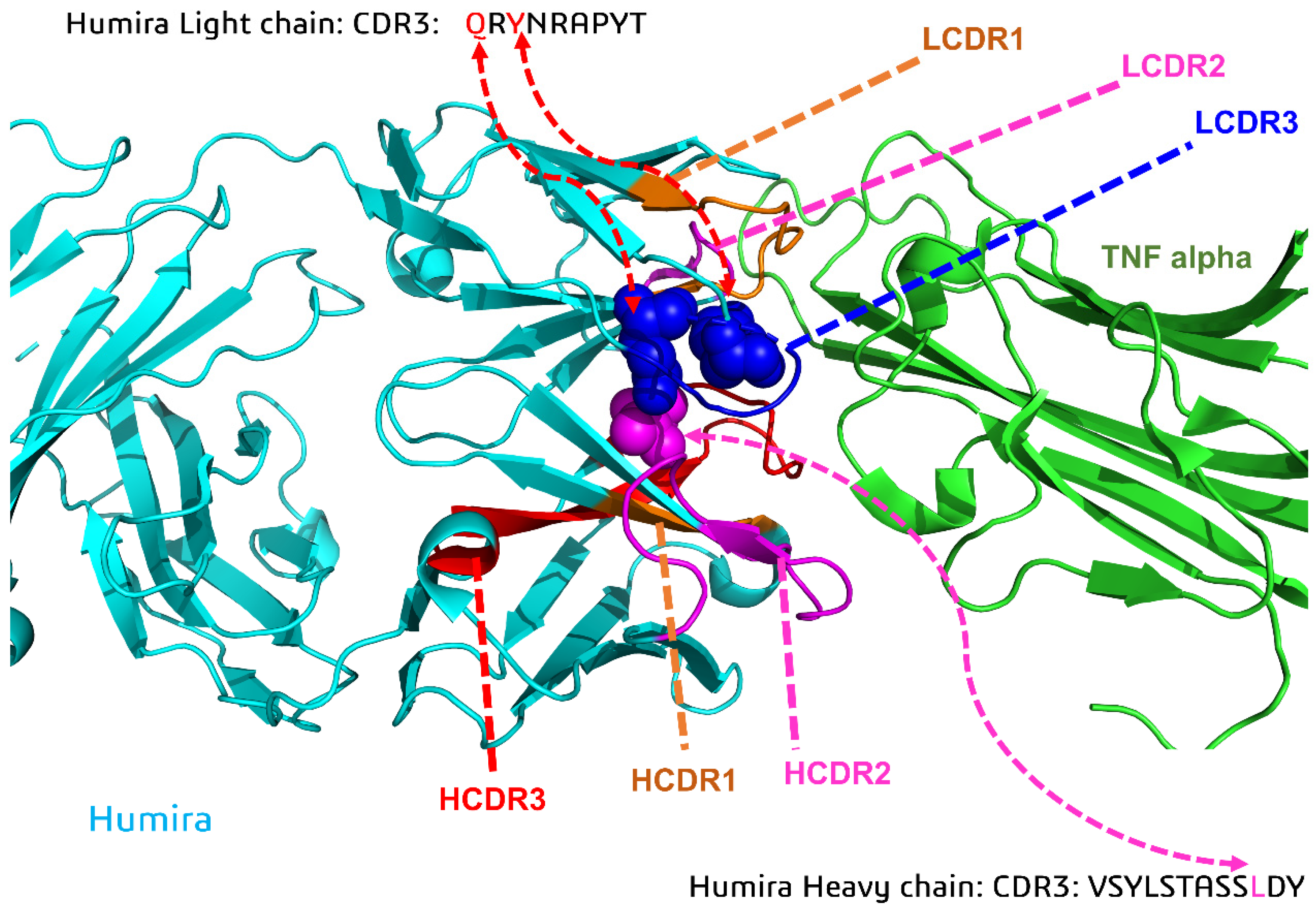
Biomolecules | Free Full-Text | Anti-TNF Alpha Antibody Humira with pH-dependent Binding Characteristics: A constant-pH Molecular Dynamics, Gaussian Accelerated Molecular Dynamics, and In Vitro Study