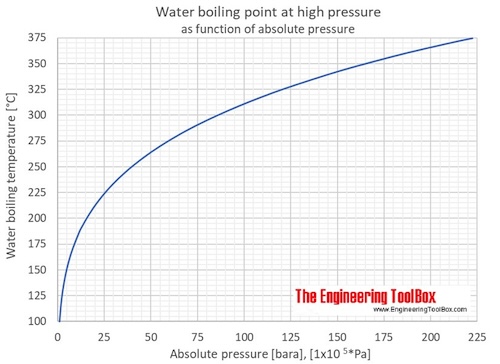
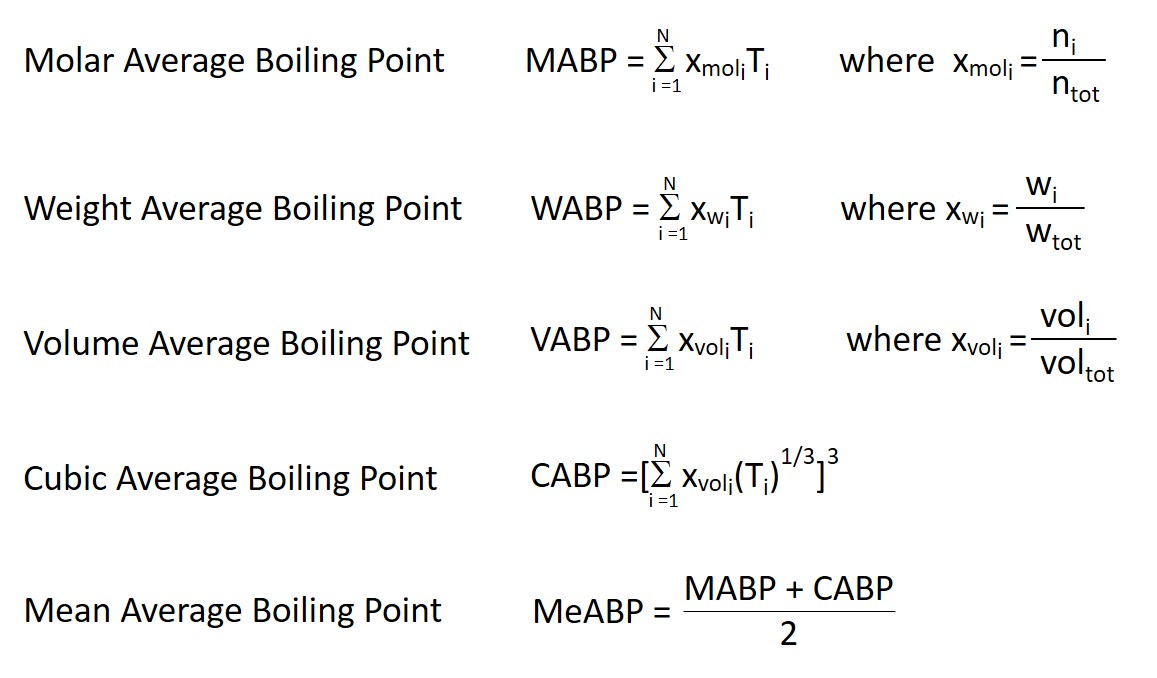
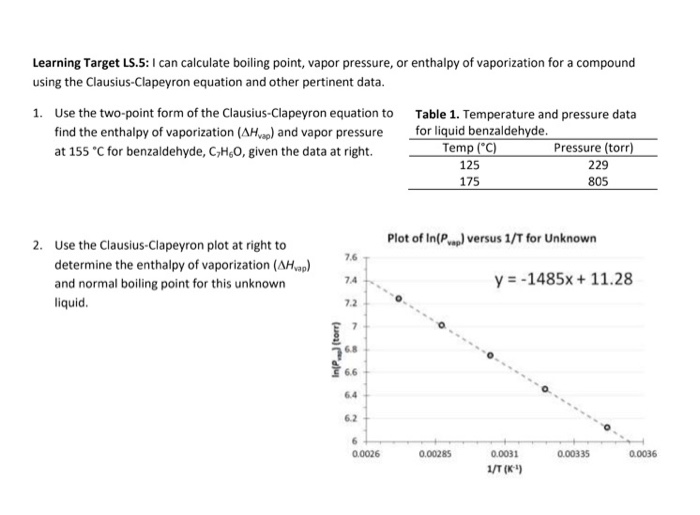
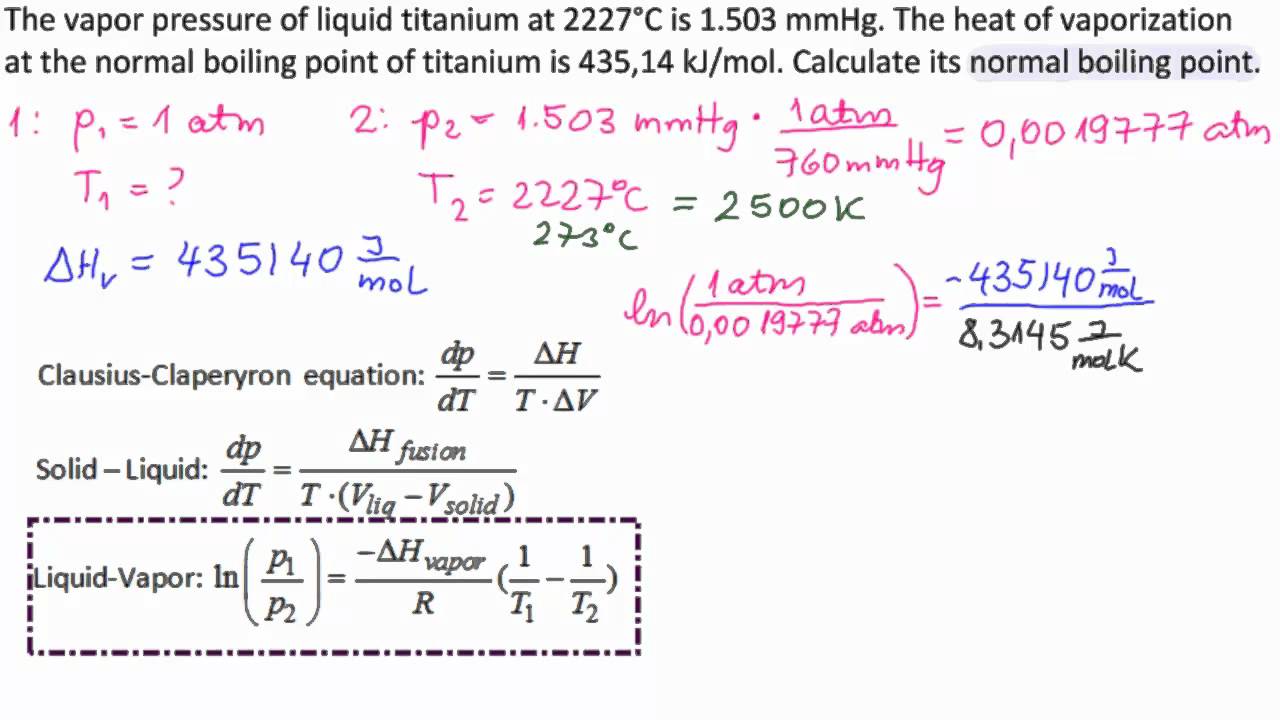
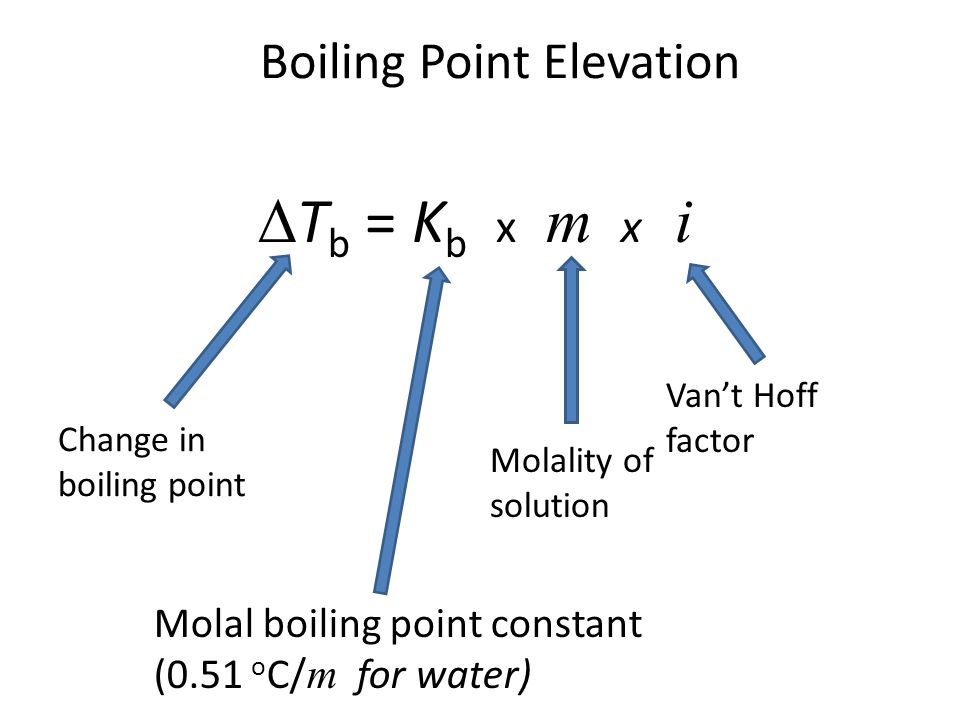
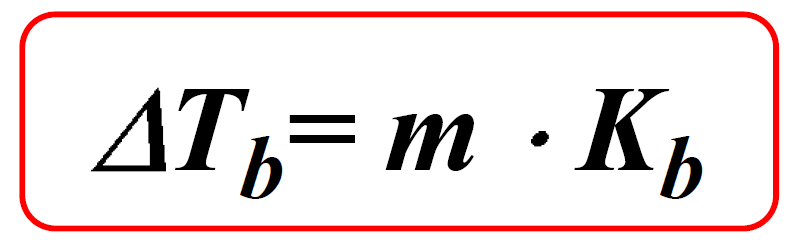Calculation the boiling point of a 1M aqueous solution (density 1.04 g mL^-1 )of potassium chloride (Kb for water = 0.52 K kg mol^-1 , Atomic masses: K = 39u, Cl =

Assuming 100% dissociation, calculate the freezing point and boiling point of 2.13 m Na_2SO_4(aq). | Homework.Study.com
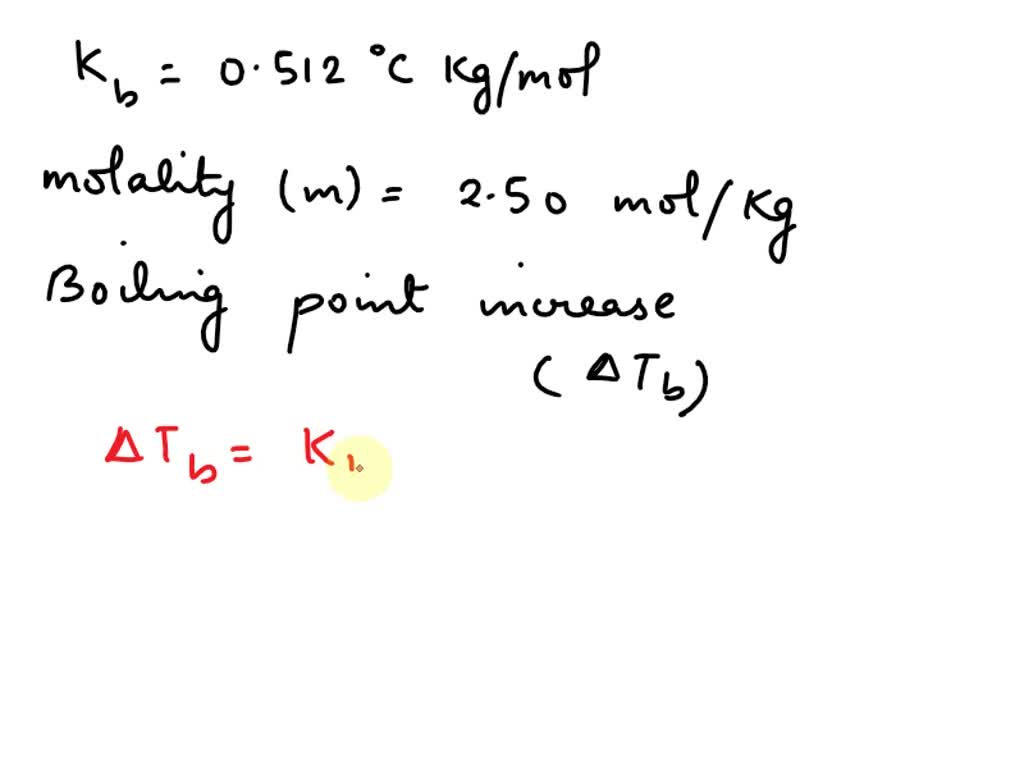
SOLVED: Given that water's boiling point is 100ºC and the elevation constant (Kb) is 0.512 ºCkg/mol, calculate the boiling point increase for a 2.50 molal solution (moles/kg) of aqueous NaCl. Assume ideal
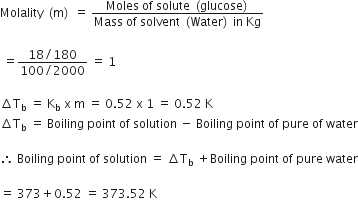
The boiling point of pure water is 373K. Calculate the boiling point of an aqueous solution containing 18 gms of glucose (MW = 180) in 100 gms of water. Molal elevation constant