Elements Hydrogen Number of: Protons 1 Neutrons 0 Electrons 1 Boiling point -252 Freezing point -259 State at room temperature gas. - ppt download
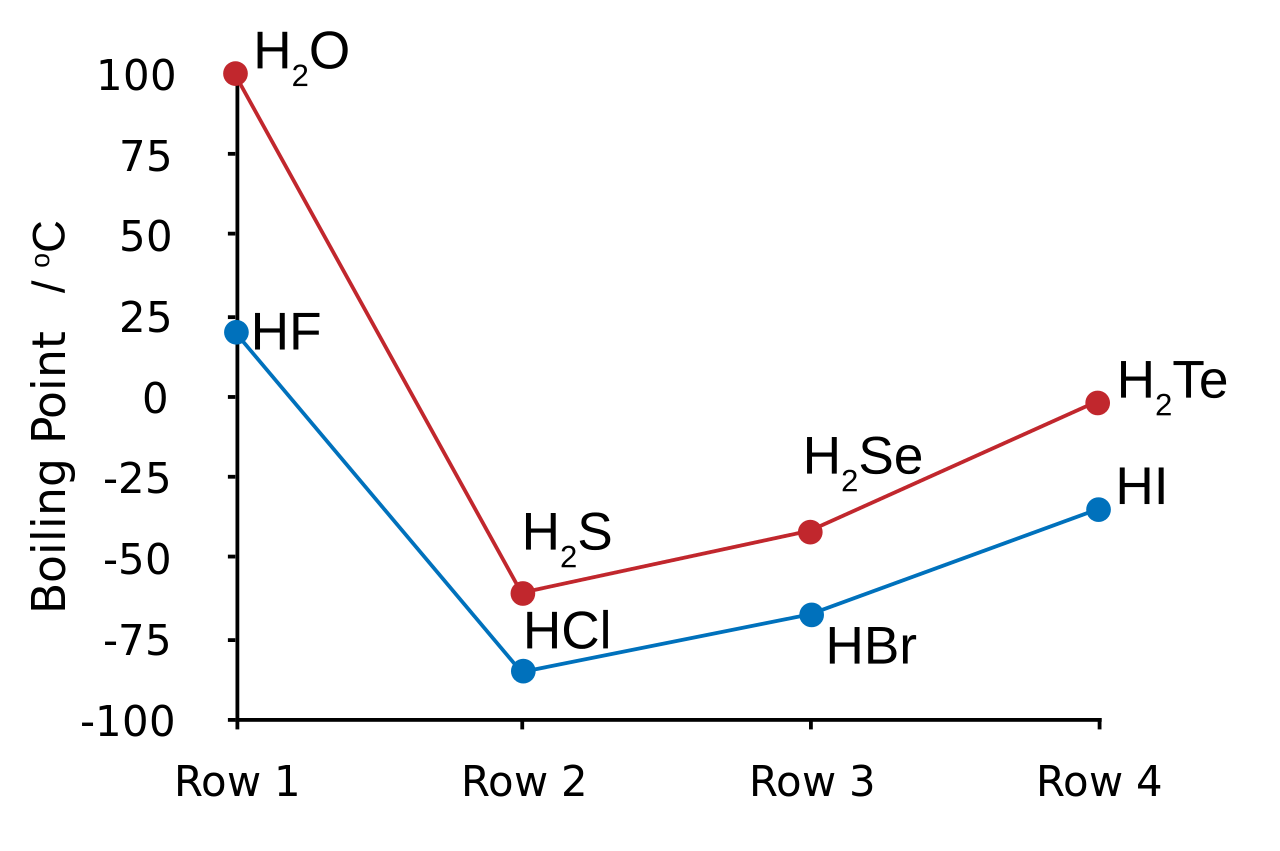
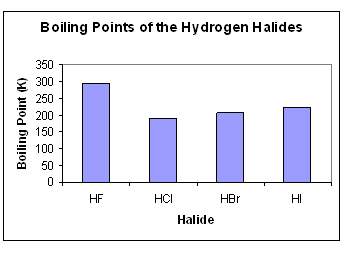
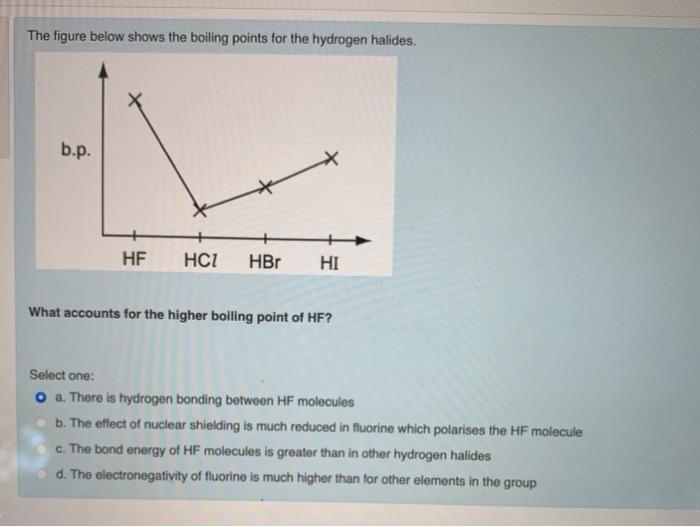
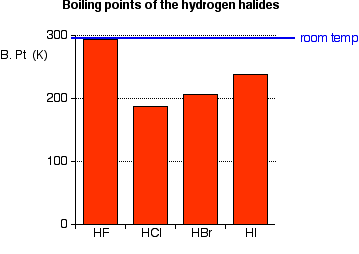
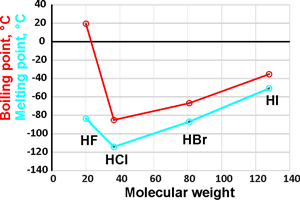
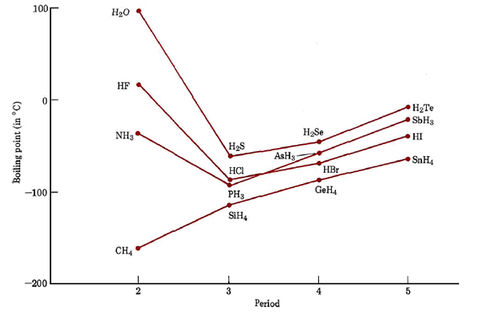
The liquefied hydrogen halides have the normal boiling points given above. The relatively high boiling point of HF can be correctly explained by which of the following? | Socratic

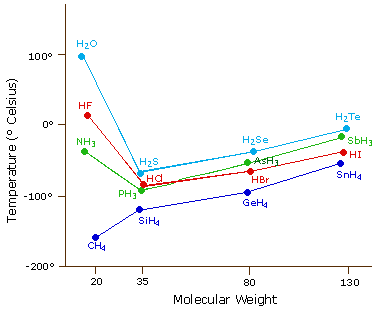
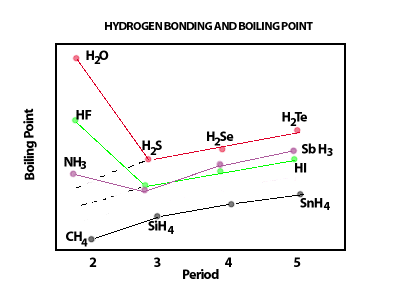
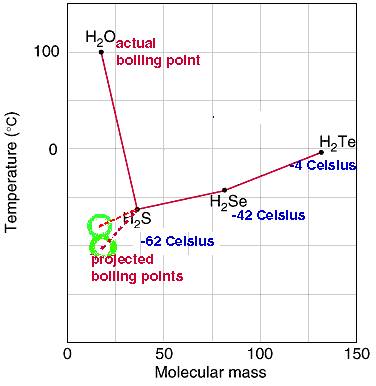
SOLVED: Q6: Unit 10 Level 2 The boiling point of water is about 2008C higher than one would predict from the boiling points of hydrogen sulfide and hydrogen selenide. One may explain
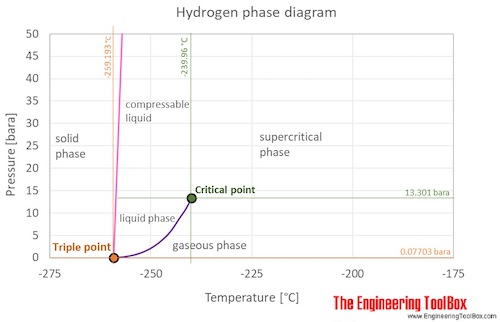
thermodynamics - Boiling point of hydrogen: relation between pressure and temperature near of triple point - Physics Stack Exchange
The effect that changes in water's hydrogen bond strength may have on... | Download Scientific Diagram

Full Frame Macro Close Up Photo of Oxygen and Hydrogen Electrolysis and Boiling Point. Stock Photo - Image of effervescence, liquid: 176172552