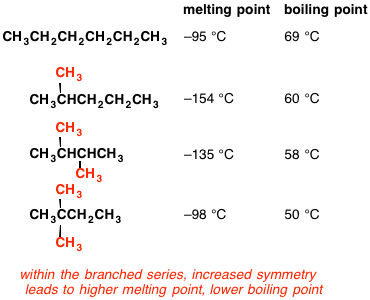
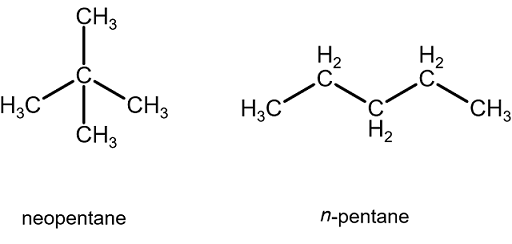
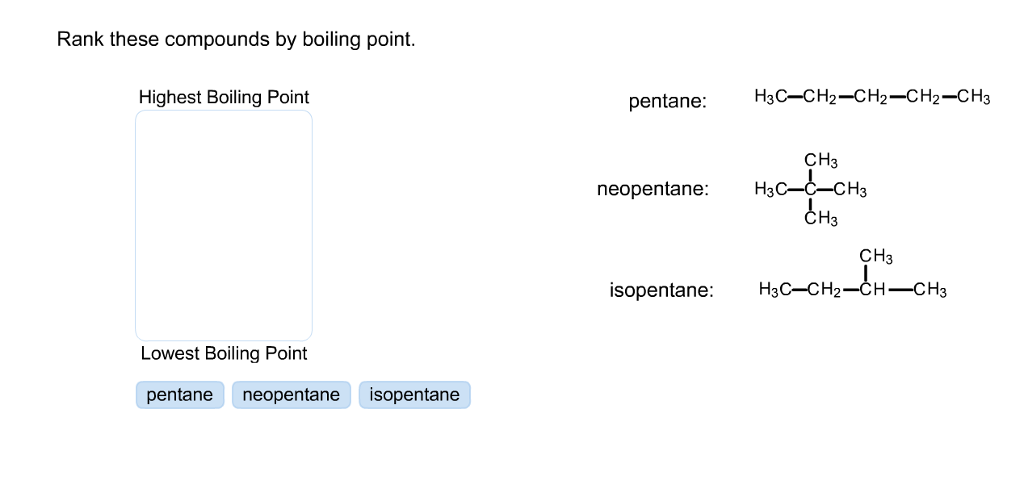
Rank these compounds from highest to lowest boiling point. a. pentane b. neopentane c. isopentane | Homework.Study.com

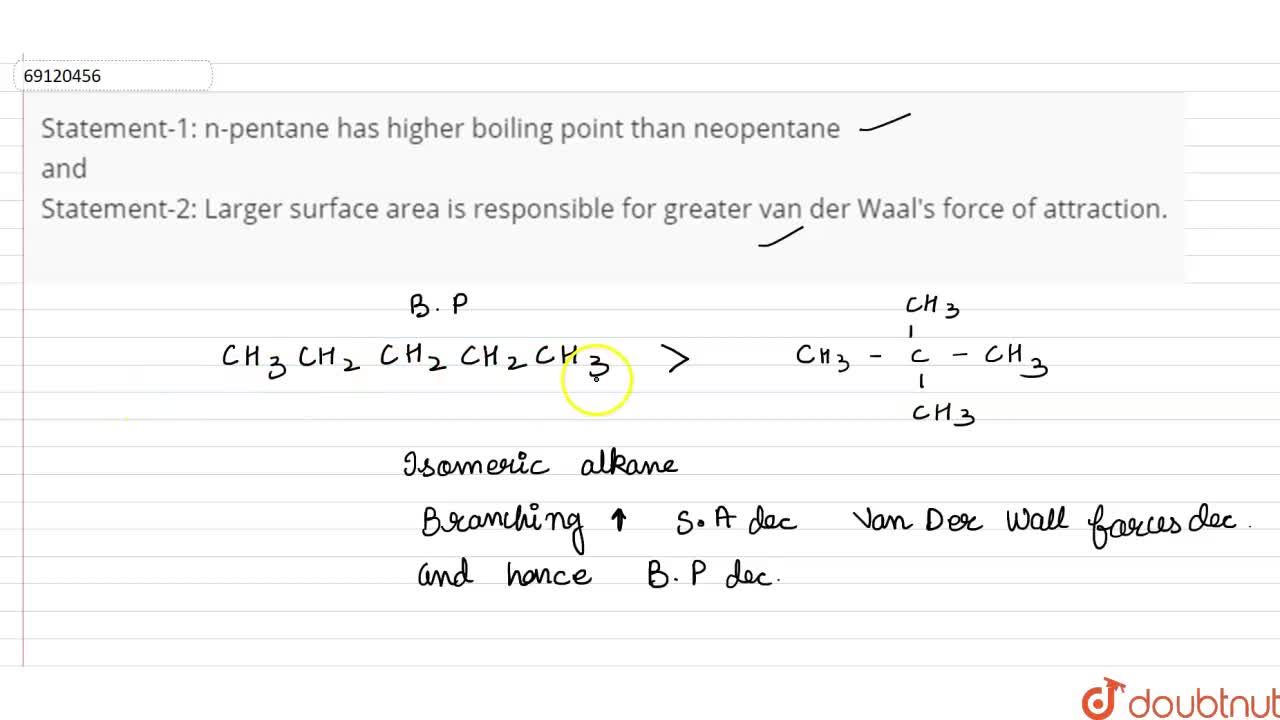
Assertion (A): Assertion A: \( n \)-Pentane has higher boiling point than neo-pentane. Reason (R... - YouTube
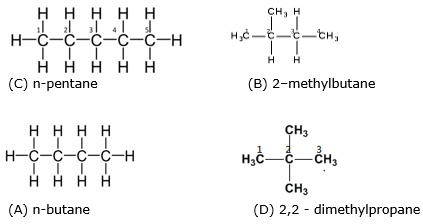
Arrange the following in decreasing order of their boiling points. (A) n–butane (B) 2–methylbutane (C) n-pentane (D) 2,2–dimethylpropane
Why Isomers of a compound have different Boiling point (like Isomers of pentane) why force of attraction is not involve in it? - Quora

organic chemistry - Why does neopentane have a higher melting point than n- pentane? - Chemistry Stack Exchange
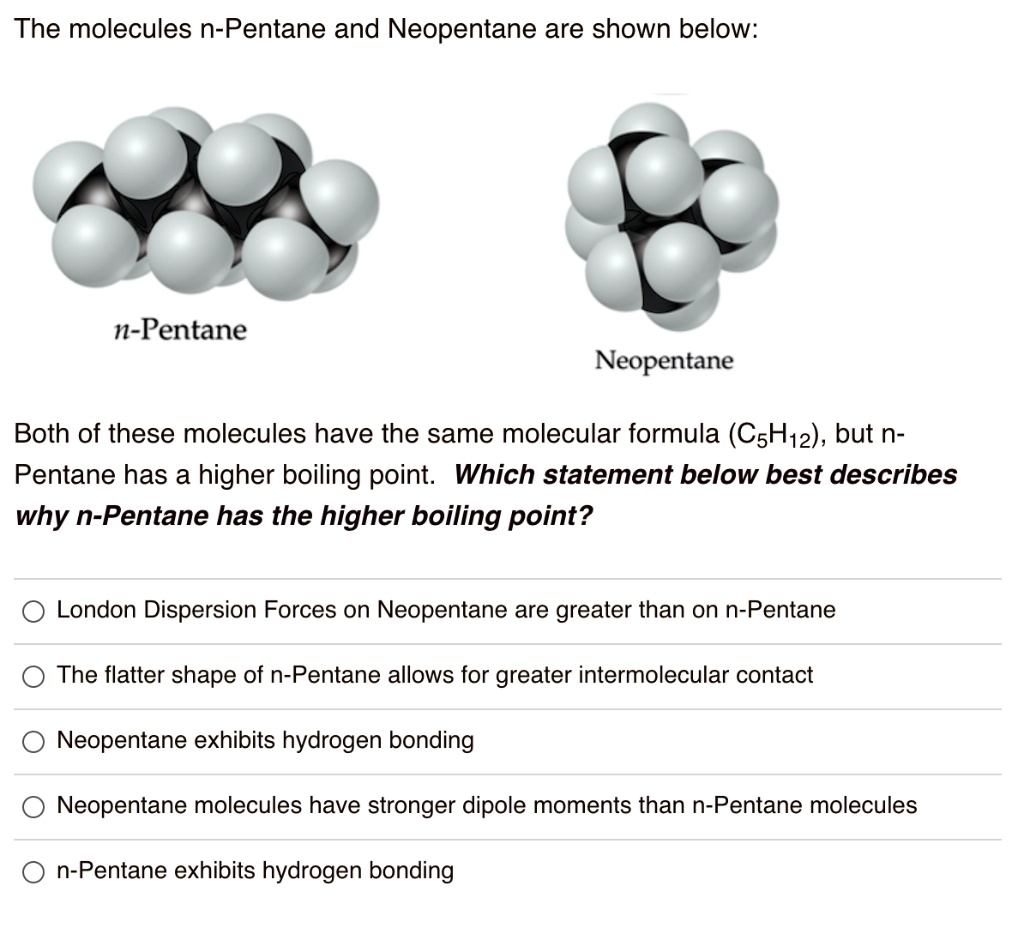
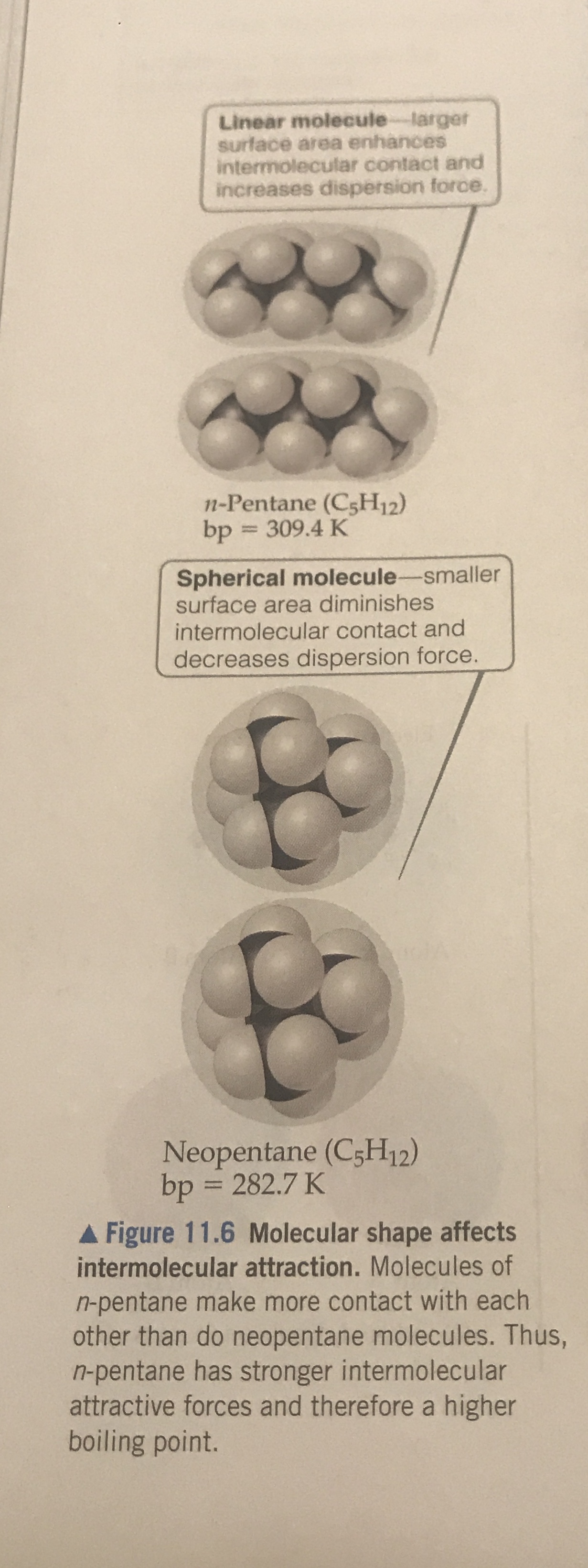
SOLVED: The molecules n-Pentane and Neopentane are shown below: n-Pentane Neopentane Both of these molecules have the same molecular formula (C5H12), but n- Pentane has a higher boiling point: Which statement below
21. Why The boiling point of pentane is greater than isopentane? And why the boiling point of neopentane is less than N pentane and isopentane?

why neopentane has higher melting point than n pentane - Chemistry - Chemical Bonding and Molecular Structure - 13416933 | Meritnation.com
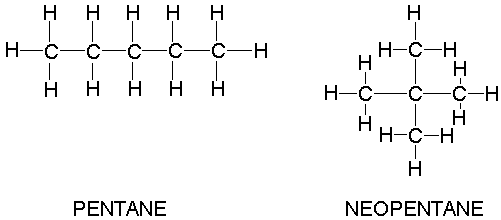
Why when the shape of molecules become more compact it's boiling point decrease while when intermolecular force become strong boiling point increase? | Socratic





![Q19E Rationalize the difference in bo... [FREE SOLUTION] | StudySmarter Q19E Rationalize the difference in bo... [FREE SOLUTION] | StudySmarter](https://studysmarter-mediafiles.s3.amazonaws.com/media/textbook-exercise-images/image_5mJYPJZ.png?X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Credential=AKIA4OLDUDE42UZHAIET%2F20230601%2Feu-central-1%2Fs3%2Faws4_request&X-Amz-Date=20230601T203606Z&X-Amz-Expires=90000&X-Amz-SignedHeaders=host&X-Amz-Signature=71b205adb6e633a296aa7e69d2344ee9fb127e3b4b05cd85d7f899ba93f67155)