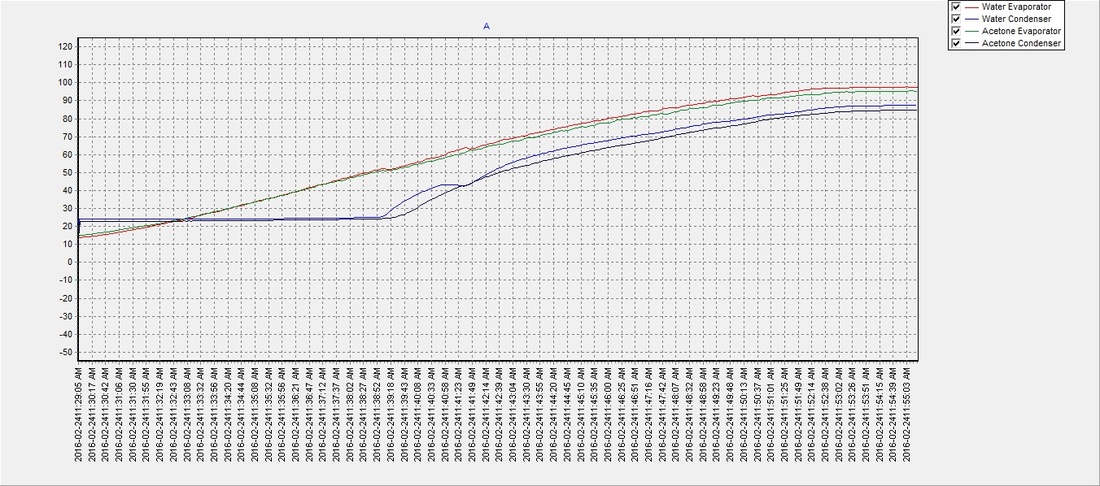
Enhancement of solvent uptake in porous PVDF nanofibers derived by a water-mediated electrospinning technique - ScienceDirect

SOLVED: Which of the following compound pairs could be effectively separated by simple distillation? Briefly explain your choice. sec-butanol and acetophenone aniline and n-hexane isopentane and benzene 0-xylene and acetone Vacuum distillation

a) Boiling temperature-solubility parameter graph with various organic... | Download Scientific Diagram
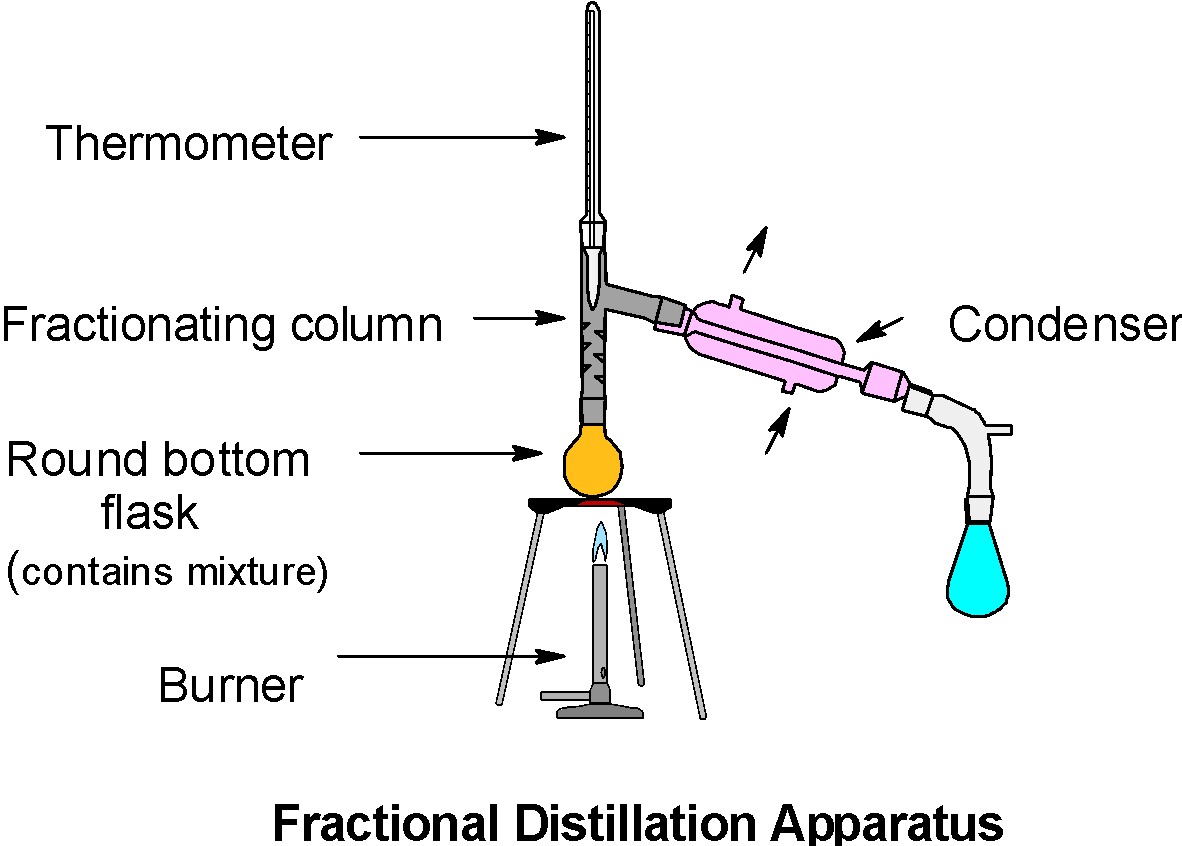
Acetone and methanol can be separated by: A) Steam distillation B) Fractional distillation C) Vacuum distillation D) None of the above